In today’s competitive medical device market, sourcing reliable hearing aid manufacturers is more important than ever. Certifications like FDA, CE, and ISO are essential indicators of a manufacturer’s credibility, quality, and compliance with international standards.
Why Certifications Matter in Hearing Aid Manufacturing
- Product quality assurance
- Legal compliance in target markets
- User safety and satisfaction
- Customs clearance and logistics
- Brand reputation and liability protection
Understanding the Three Key Certifications
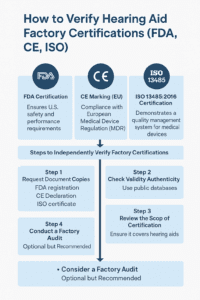
1. FDA Certification
What It Means: The FDA ensures that hearing aids sold in the U.S. meet specific safety and performance requirements.
How to Verify:
- Visit the FDA database
- Search by manufacturer’s name or product code
2. CE Marking (EU)
What It Means: A CE mark indicates compliance with EU Medical Device Regulation (MDR).
How to Verify:
- Request the EC Declaration of Conformity
- Check Notified Body number in the NANDO database
3. ISO 13485:2016 Certification
What It Means: Demonstrates a quality management system for medical devices, including hearing aids.
How to Verify:
- Request the ISO certificate
- Check issuing body via IAF CertSearch
Steps to Independently Verify Factory Certifications
- Request Document Copies: FDA registration, CE Declaration, ISO certificate
- Check Validity: Cross-check with public databases
- Review Scope: Ensure it covers hearing aid production
- Consider Factory Audit: Optional but highly recommended for bulk orders
Common Red Flags in Certification Documents
| Red Flag | What It Means | Action to Take |
|---|---|---|
| Generic product category | Not certified specifically for hearing aids | Request correct scope |
| Missing expiration date | Certificate may be outdated or invalid | Ask for latest version |
| Unknown certifying body | Possibly a fake or non-accredited issuer | Check IAF/NANDO |
| Supplier avoids document sharing | Lack of transparency or non-compliance | Reconsider supplier |
How Goodmi Ensures Full Certification Transparency
At Goodmi Intelligent (Shenzhen) Co., Ltd., we provide:
- FDA-registered hearing aids
- CE-compliant under MDR standards
- ISO 13485:2016 certified production facility
All documents are available upon request, and we support third-party audits at our Shenzhen factory. As a trusted OEM/ODM partner, we help ensure your hearing aid supply chain is fully compliant.
Conclusion
Verifying FDA, CE, and ISO certifications should be part of every hearing aid procurement manager’s due diligence checklist. Doing so not only safeguards your reputation but ensures legal compliance and consistent quality for your markets.
Need help verifying your hearing aid supplier?
Contact Goodmi today or download our certification checklist to simplify your sourcing process.




